Cardiac Safety Assays
We study the effects of compounds (and biologics), whatever their therapeutic areas for various purposes: mandatory safety, toxicology study, secondary pharmacology or integrated assessments during drug discovery phases.
Cardiac Ion Channel screening/activity
Human iPSC CM-Isolated Perfused Heart
Vascular animal models
Others
We offer a wide selection of ion channel assays as well as additional cardiac safety studies in compliance with all ICH S7A and S7B guidelines and Good Laboratory Practices. We are a Preclinical Contract Research Organization of highly experienced cardiovascular pharmacology and electrophysiology experts. As preclinical cardiovascular expert, PhysioStim provides a wide range of possibilities to conduct both your mandatory and complementary ion channel assays for reliable and efficient cardiac safety studies. We also respect the 3R principle and have already taken steps to comply with the upcoming CIPA guidelines.
Cardiac Ion Channel Screening
/ CiPA Seven Ion Channel screening
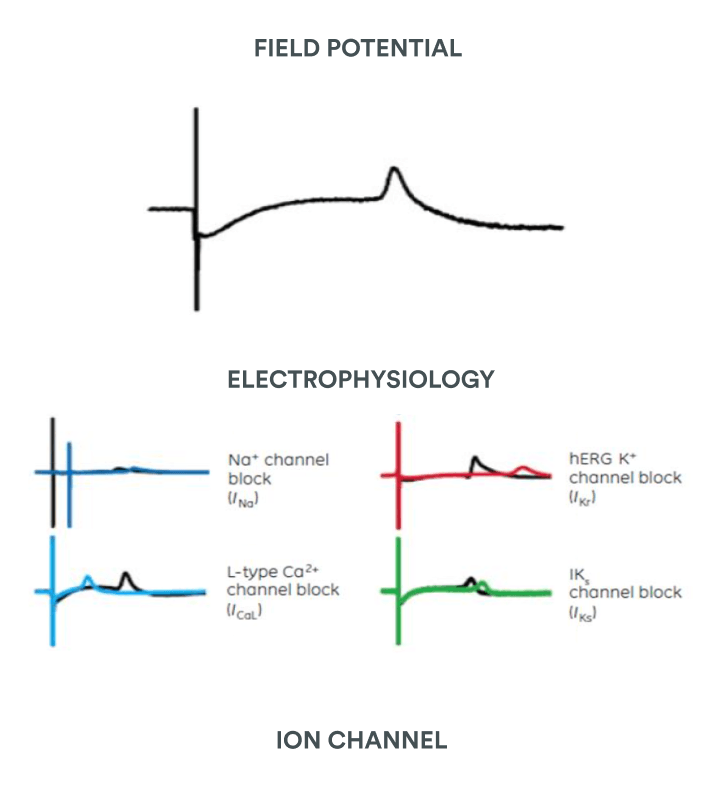
PhysioStim provides assays to test your compounds on CiPA cardiac ion channels panel using the automated or manual patch clamp technique following the last regulatory ICH E14/S7B Q&A (2022) recommendations.
One of the main points of CiPA is the profiling of the compounds against a range of cardiac ion channels
All the “CiPA seven” ion channels: hERG* | hNav1.5 peak* | hCav1.2 * | hKvLQT1/MinK | hKir2.1 | hKv4.3 | hNav1.5 late
* = can also be performed under GLP conditions with manual patch clamp technique
New cardiac safety guidelines being developed by the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative from expert working groups: FDA, HESI (Health and Environmental Sciences Institute) and CSRC (Cardiac Safety Research Consortium). CiPA initiative aims to improve the existing FDA regulations (ICH S7B and ICH E14) by requesting new guideline for assessment of the proarrhythmic potential of new drugs with improved specificity compared with traditionnal hERG assay + Thorough QT study.
With a dedicated expertise in cardiovascular pharmacology and electrophysiology from 2001, PhysioStim offers a comprehensive CiPA model and can assist you at each step of your project.
/ Cardiac Ion Channel package
Automated Patch Clamp Platform
With our automated Patch Clamp platform we provide a high quality IC50 values ! Choose to get premium data with our high-quality automated patch palteform to study ion channels at early stage of development.
We recommend to de-risk your compounds with our CARDIAC ION CHANNEL SCREENING PACKAGE
Due to the importance of the 3 main cardiac ion channels (Nav1.5, Cav1.2, & hERG) in the cardiac electrophysiology (shape of ventricular action potential & ECG morphology), we recommend testing these 3 ion channels in an early screening procedure
/ GLP hERG assay
Preclinical hERG assays aim to identify potential blockade effects early in drug discovery phase. ICH Guidelines state that hERG assays are mandatory, due to numerous clinically approved drugs reported to increase the pro arrhythmogenic risk (including sudden death) through hERG channel blockade. As a result, a large number of drugs have been withdrawn during late stage of clinical trials or from the market. By running this assays, you’ll be able to determine your safety margin.
Dose formulation analysis is a mandatory requirement for GLP studies submitted in IND filings PhysioStim offers the possibility to include and manage this analytical work with GLP accredited PhysioStim’s partners.
Our expertise in a few numbers
90%
of GLP hERG projects are performed under E14/S7B Q&A recommendations
+200
GLP hERG projects in total conducted in 20 years
64 % of the last 200 compounds tested in a hERG non-GLP study performed at PhysioStim have shown at least 30% inhibition on hERG channel at their highest concentration 43 % of the last 100 compounds tested in a hERG GLP study performed at PhysioStim have shown at least 30% inhibition on hERG channel at their highest concentration
Human iPSC Cardiomyocytes
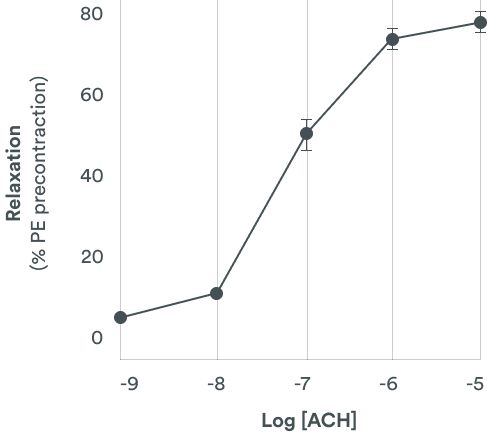
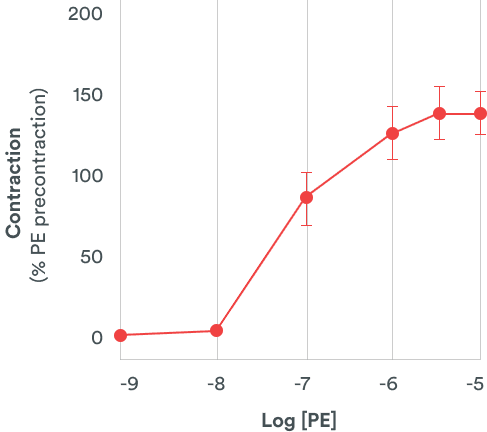
Human cardiomyocytes derived from induced pluripotent stem cells (hiPSC-CM) provide rapid, reproducible and sensitive predictions of QT prolongation. Besides QT prolongation, it is also an integrative system that could easily identify potential cardiotoxic effects of compounds (pro-arrhythmogenic, cytotoxic and contractility worsening).
Human Cardiomyocytes assays with a three-in-one assay
This model is relevant to investigate series of compounds that could support the ranking selection of candidates based on human pharmacology.
/ Human Cardiomyocytes assays
- Primary screening assays
- Cardiotoxicity evaluation and inotropism
- CiPA assays for proarrhythmic liabilities (prediction of QT prolongation)
Platform
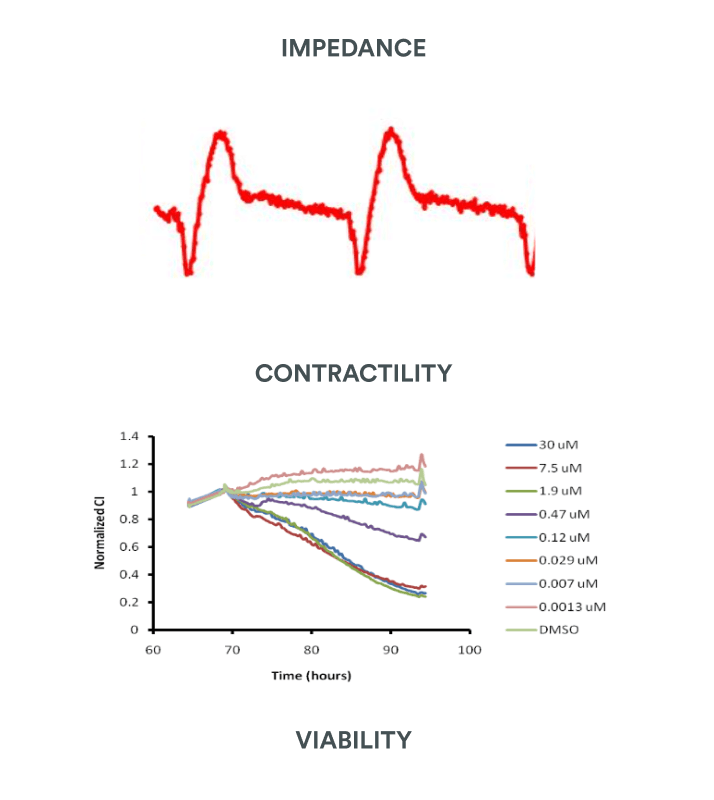
/ Impedance : contractility & cell viability
- Amplitude of contraction
- Beating rate & beating period
- Amplitude of contraction
- Individual beat duration (IBD10, 50, 90)
- Proarrhythmic events (BRI)
- Cell Index
/ MEA: electrophysiology
- FPD and FPDc intervals (Fridericia) mimics ECG
- Spike amplitude
/ Biomarkers
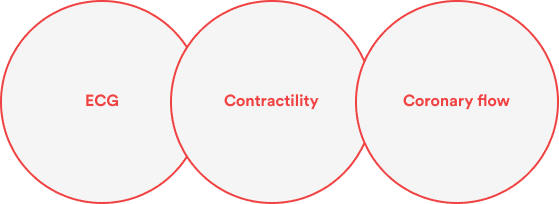
Isolated perfused heart
The isolated perfused heart assay provides rapid, reproducible and sensitive predictions of QT prolongation as recommended by ICH S7B guideline. This is also an integrative and native expressed system that could easily identify potential cardiotoxic effects of compounds (pro-arrhythmogenic, cytotoxic and contractility worsening).
This model can be used as a screening procedure as well as it can be conducted under GLP conditions with analytical analysis.
Our expertise in a few numbers
≈150
projects already performed on isolated perfused heart model (GLP compliant or not)
+200
compounds tested in total in 20 years (in GLP or not GLP-conditions)
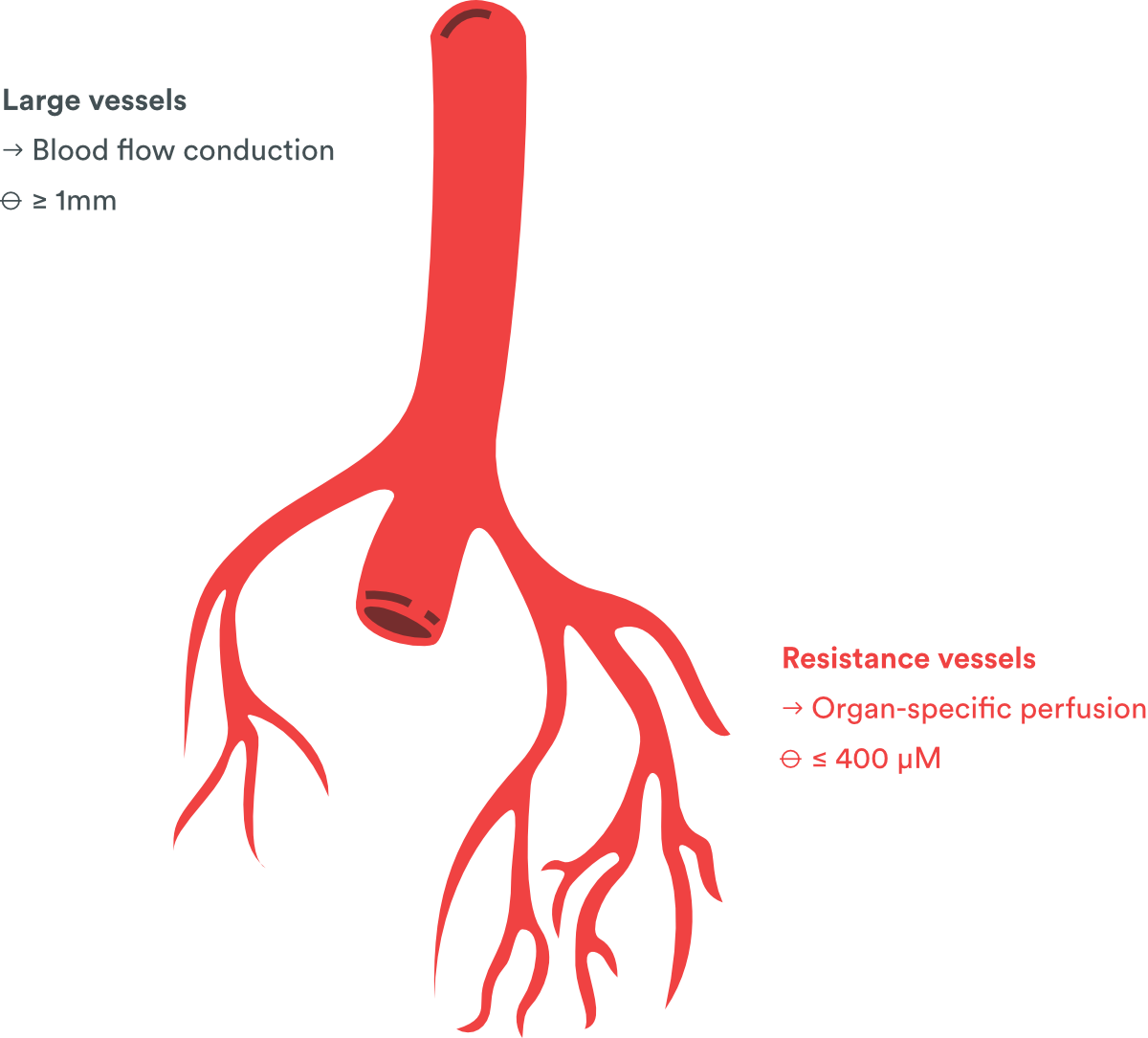
Vascular assays (animal models)
Due to on-target or off-target effects, numerous clinically approved drugs are reported to modulate vascular hemodynamics. In this context, vascular reactivity study allows to:
- Identify potential detrimental hemodynamic effects early during drug development (“Derisking”)
- Identify of therapeutic potential on vascular function during drug development (Exploration and innovation)
By the use of isolated organ bath or wire myograph, PhysioStim offers the opportunity to study a wide range of vascular type, from large vessels (aorta, pulmonary artery, carotid artery…, in isolated organ bath set-up) to resistance vessels (model of mesenteric resistance artery in myographs set-up) in a wide range of animal species (rat, guinea-pig, mouse, rabbit).
Cardiac telemetry
Cardiovascular monitoring methods using telemetry have become an important component in preclinical safety assessments over the last decade, providing pharmacologists a simple but valuable tool to evaluate drug safety.
PhysioStim proposed telemetry in rodent to monitor a variety of cardiovascular parameters such as blood pressure, heart rate (HR), electrocardiogram (ECG), and body temperature (BT), amongst others, in awake, and freely moving laboratory animals. These systems are typically employed in regulatory safety pharmacology studies as detailed in the S7A guideline from the International Conference of Harmonization (ICH) (FDA, 2001).
Purkinje Fiber
Action potential from Purkinje fibers provide a reproducible and sensitive predictions of QT prolongation. Thus the ionic channels involved in the Purkinje action potenial shape are similar to those of the human ventricular repolarization. Therefore any lengthening of action potential duration (APD) are highly predictible of human QT prolongation and incidence of ventricular arrhythmias. Among species and cardiac tissues, action potential recordings in female rabbit Purkinje fibers constitute the most sensitive model to detect the effects of hERG blocking agents on cardiac repolarization. Indeed, inhibition of potassium currents contributing to the terminal phase of repolarisation of the cardiac action potential (IKr / IKs and IK1) is a mechanism by which agents increase APD.